Invest. Support. Grow.
Nueterra Capital was founded to empower entrepreneurs — to equip innovative minds with the capital, resources and expertise to help drive change. We provide financial investment as well as access to a network of resources to support our portfolio companies and create wealth for our investors.
Mission
Innovation is Our Bottom Line
Nueterra Capital invests in early and growth-stage companies that are focused on changing the status quo. We equip innovative minds with the capital, resources, and expertise to catapult their growth, create wealth for our investors, and help drive transformation across a range of industries.
Investment Strategy
We Capitalize on Change
Our investment strategy is focused on identifying untapped opportunities for wealth creation. We believe the types of problems businesses and consumers face are changing, and with that change comes significant opportunities for companies that can innovate quickly. We invest in companies that embrace change as a means of economic growth and demonstrate a commitment to their vision.
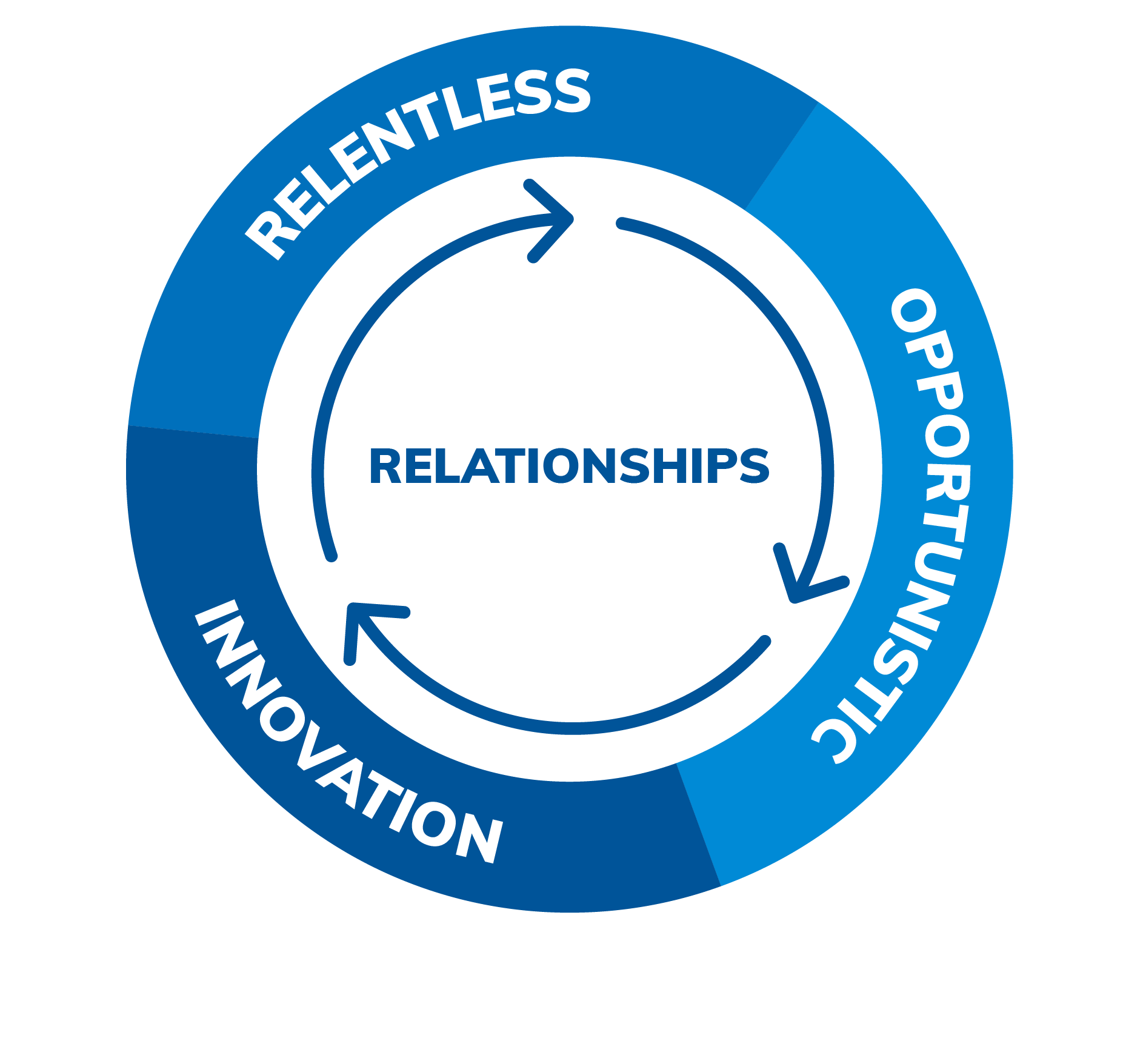
Our Values
Our Team
The Nueterra Capital team is comprised of experienced entrepreneurs and finance professionals who understand what it’s like to be a founder – and how to capitalize on that experience to create wealth for our investors.

Dan Tasset
Chairman

Jeremy Tasset
Chief Executive Officer

Mark Jones
Chief Financial Officer

Emmanuel Emah-Emeni
Managing Director

Travis Tasset
Senior Vice President
